AZD 9977
CAS No. 1850385-64-6
AZD 9977( AZD9977 )
Catalog No. M12858 CAS No. 1850385-64-6
AZD9977 is a novel, first-in-class, selective and oral mineralocorticoid receptor (MR) modulator with pKi of 7.5, shows little to no affitnity for GR, PR and AR (pIC50<5.5).
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 259 | Get Quote |


|
| 10MG | 430 | Get Quote |


|
| 25MG | 710 | Get Quote |


|
| 50MG | 972 | Get Quote |


|
| 100MG | 1332 | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameAZD 9977
-
NoteResearch use only, not for human use.
-
Brief DescriptionAZD9977 is a novel, first-in-class, selective and oral mineralocorticoid receptor (MR) modulator with pKi of 7.5, shows little to no affitnity for GR, PR and AR (pIC50<5.5).
-
DescriptionAZD9977 is a novel, first-in-class, selective and oral mineralocorticoid receptor (MR) modulator with pKi of 7.5, shows little to no affitnity for GR, PR and AR (pIC50<5.5); dose dependently reduces albuminuria and improves kidney histopathology similar to eplerenone in db/db uni-nephrectomised mice and uni-nephrectomised rats, does not affect urinary Na+/K+ ratio.Diabetes Phase 1 Clinical(In Vitro):Balcinrenone (AZD9977) and eplerenone activities on MR, GR, PR and AR in binding assays. The observed pKi of MR, GR, and PR are 7.5, 5.4 and 4.6, respectively.Functional interaction of Balcinrenone with MR is characterized in a reporter gene assay where the full-length MR drives a luciferase reporter gene in U2-OS cells. Balcinrenone antagonizes aldosterone-activated MR with an IC50 of 0.28 μM. Whereas eplerenone is a full antagonist, Balcinrenone suppresses only 69% of the MR activity in this assay.Species selective potencies of Balcinrenone are established in reporter gene assays using the MR LBDs from human, mouse or rat. The corresponding IC50 values are 0.37 μM, 0.08 μM and 0.08μM, respectively. (In Vivo):Balcinrenone (AZD9977) (oral administration; 10-100 mg/kg; 4 weeks) dose dependently reduces the UACR compared to vehicle in uni-nephrectomised male Sprague Dawley rats administered aldosterone and fed a high-salt diet. Balcinrenone is as efficacious as full MR antagonists on renal protection, despite the partial antagonism observed in in vitro assays.Balcinrenone (oral administration; 100 mg/kg; co-administration with enalapril) stops further disease progression and reduces the urine albumin excretion (UAE) compared to vehicle treatment. Co-administration of enalapril has an apparent additive effect on UAE reduction, although this reduction is not statistically significant.
-
In VitroBalcinrenone (AZD9977) and eplerenone activities on MR, GR, PR and AR in binding assays. The observed pKi?of MR, GR, and PR are 7.5, 5.4 and 4.6, respectively.Functional interaction of Balcinrenone with MR is characterized in a reporter gene assay where the full-length MR drives a luciferase reporter gene in U2-OS cells. Balcinrenone antagonizes aldosterone-activated MR with an IC50 of 0.28 μM. Whereas eplerenone is a full antagonist, Balcinrenone suppresses only 69% of the MR activity in this assay.Species selective potencies of Balcinrenone are established in reporter gene assays using the MR LBDs from human, mouse or rat. The corresponding IC50 values are 0.37 μM, 0.08 μM and 0.08μM, respectively.
-
In VivoBalcinrenone (AZD9977) (oral administration; 10-100 mg/kg; 4 weeks) dose dependently reduces the UACR compared to vehicle in uni-nephrectomised male Sprague Dawley rats administered aldosterone and fed a high-salt diet. Balcinrenone is as efficacious as full MR antagonists on renal protection, despite the partial antagonism observed in?in vitro?assays.Balcinrenone (oral administration; 100 mg/kg; co-administration with enalapril) stops further disease progression and reduces the urine albumin excretion (UAE) compared to vehicle treatment. Co-administration of enalapril has an apparent additive effect on UAE reduction, although this reduction is not statistically significant. Animal Model:Uni-nephrectomised male Sprague Dawley rats administered aldosterone and fed a high-salt diet with AZD9977 Dosage:10, 30 and 100 mg/kg Administration:Oral administration; 10-100 mg/kg; 4 weeks Result:Improved kidney function and histology in animal models of CKD.Animal Model:Db/db mice uni-nephrectomised at 8 weeks of age are treated from age 18w to age 22w Dosage:100 mg/kg Administration:Oral administration; 100 mg/kg; co-administration with enalapril Result:Reduced albuminuria in diabetic kidney disease.Co-administration of enalapril with AZD9977 had an additive effect on renal pathology scoring.
-
SynonymsAZD9977
-
PathwayNuclear Receptor/Transcription Factor
-
TargetMLR
-
RecptorMLR
-
Research AreaMetabolic Disease
-
IndicationDiabetes
Chemical Information
-
CAS Number1850385-64-6
-
Formula Weight399.378
-
Molecular FormulaC20H18FN3O5
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 250 mg/mL (625.99 mM)
-
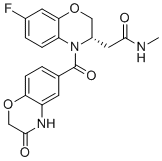
SMILESCNC(=O)CC1COC2=C(N1C(=O)C3=CC4=C(C=C3)OCC(=O)N4)C=CC(=C2)F
-
Chemical Name(S)-2-(7-fluoro-4-(3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-6-carbonyl)-3,4-dihydro-2H-benzo[b][1,4]oxazin-3-yl)-N-methylacetamide
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Bamberg K, et al. PLoS One. 2018 Feb 23;13(2):e0193380.
2. Erlandsson F, et al. Br J Clin Pharmacol. 2018 Feb 21. doi: 10.1111/bcp.13562.
molnova catalog



related products
-
Finerenone
Finerenone (BAY 94-8862) is a potent, selective, orally available mineralocorticoid receptor (MR) antagonist with IC50 of 18 nM.
-
KBP-5074
KBP-5074 (KBP5074) is a novel non-steroidal, highly selective mineralocorticoid receptor antagonist for the treatment of hypertensive chronic kidney disease.
-
CS-3150
CS-3150 (Esaxerenone, XL-550) is a?highly potent, selective and orally active non-steroidal mineralocorticoid receptor antagonist with IC50 of 9.4 nM.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


